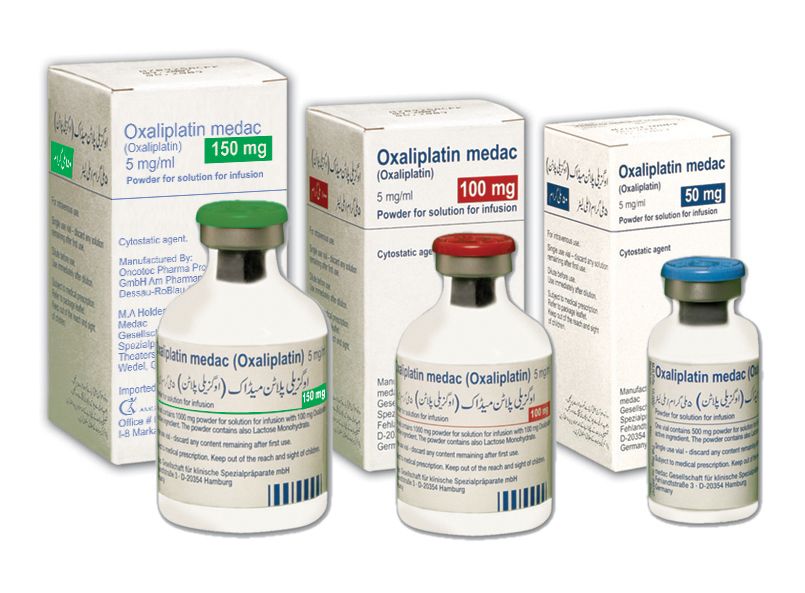
Oxaliplatin Medac (oxaliplatin for injection) is an antineoplastic agent for the treatment of colorectal cancer. Oxaliplatin is an organoplatinum complex in which the platinum atom is complexed with 1, 2-diaminocyclohexane (DACH) and with an oxalate ligand as a leaving group.
Oxaliplatin Medac is supplied in vials containing 50mg, 100mg & 150mg of oxaliplatin as a sterile, preservative-free lyophilized powder for reconstitution. Oxaliplatin medac is a 3rd generation platinum compound with wide antitumor effect & have better safety profile than cisplatin. There is lack of cross resistance with cisplatin or carboplatin.
As First line therapy for advanced colorectal carcinoma. Oxaliplatin medac, used in combination with infusional 5-FU/LV, is indicated for the treatment of patients with metastatic carcinoma of the colon or rectum whose disease has recurred or progressed during or within 6 months of completion of first-line therapy with the combination of bolus 5-FU/LV and irinotecan.
• Standard adjuvant therapy for stage II & III Colon Cancer [8]
• Results into dramatic reduction in risk of recurrence [9]
• Has better efficacy & tolerability profile
• Offers reduced hematologic toxicity
Ref: [8] Oncolog, October 2010, Vol 55, No 10
[9] Annals of Oncology 16 (supplement 2) ii33-ii140, 2005
Thrombocytopenia may compromise cancer treatment, causing chemotherapy dose reductions, schedule alterations, or the need for platelet transfusions.
Read more
Colorectal cancer is a major cause of morbidity and mortality, being one of the most common malignant tumors in the world.
Read more
Cancer of the prostate is the most common malignancy in elderly men
Read more
Chemotherapy-induced neutropenia is a common and serious complication of myelosuppressive chemotherapy. It is associated with significant morbidity and mortality, and can increase the overall cost of providing cancer therapy.
Read more
Some types of cancer may cause bone damage leading to high levels of blood calcium and lesions that cause severe bone pain, bone masses & fractures.
Read more